Unlike leukemic driver mutations, PIGA mutations produce an escape phenotype in the context of immune-mediated bone marrow failure such as aplastic anemia (AA). Another way to create clinical advantage will be to disable HLA-mediated cytotoxic T cell recognition. Determinants of cytotoxic T cell response might include some accessories glycosylphosphoinositol (GPI)-linked moieties but the main stimulus is likely to be provided by HLA-presented antigenic peptides. Somatic hits in HLA genomic region (microdeletions, uniparental disomies [UPDs] of HLA locus on 6p and later mutations) have been previously assessed in AA patients [1-3]. Mechanistic analogy to immune-privileged GPI-anchor protein deficiency in PNH due to PIGA mutations[4] or deletion[5] of PIGA locus are obvious. We stipulate that HLA mutations may contribute to the intrinsic expansion of PNH clones under immune pressure being: i) additive to the effects of PIGA mutations in creating immune escape or ii) redundant and thus less frequent in PNH clones as in patients without PIGA mutation.
Using a deep targeted-sequencing panel covering HLA classical loci, and applying an in-house newly developed pipeline for the study of the HLA region (AbstractID#142501), we detected class I/II HLA somatic mutations of 10 patients with PNH. An integrative mutational analysis of PIGA and myeloid genes was then performed in order to comprehensively evaluate the role of HLA somatic hits within the scenario of PNH clonal evolution.
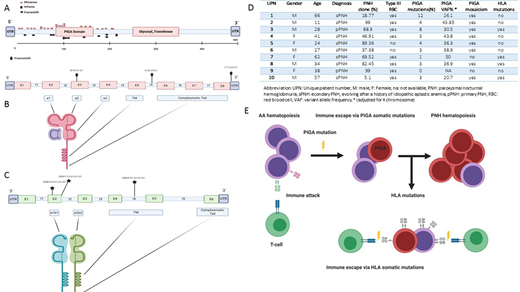
At the time of this submission HLA sequencing was completed for a total of 35 patients but full analysis is available for the first 10 cases. Overall, of these 10 PNH patients 20 samples were analyzed from sorted GPI(+) and GPI(-) myeloid fractions (mean purity >95%). Median age at diagnosis was 36 years (11-66) while median PNH granulocyte clone size at time of sampling was 76% (5.11-99). A total of 41 PIGA mutations (Fig.1A) were detected solely in the GPI(-) fraction (mean VAF 58%), with 8 patients harboring clonal mosaicism as previously described.[6] Six somatic mutations of HLA class I (N=3, Fig.1B) and class II (N=3, Fig.1C) loci were found in 4 patients (67% detected on GPI(+) and 33% on GPI(-) fraction) at a low VAF (mean 3.36%). All these events were insertions or deletion of one or more bases. Class I mutations were located in intron 5, exon 3 and 3' untranslated regions (UTR). Class II were found instead in exon 2 (N=2) and intron 4. A functional and topographical annotation based on IPD-IMGT/HLA database suggested that exonic mutations were disruptive, impairing the bio-functionality of antigen presentation site. The detected intronic mutations instead impair HLA moiety assembling within cellular membrane, possibly altering splicing of the transmembrane domain. Moreover, a computational prediction of the regulatory domains involved in the 3'UTR aberration, showed a possible involvement of the miRNA has-miR-4524a-3p binding site, potentially affecting HLA post-transcriptional regulation. Of note, in 1 patient (UPN 9, Fig.1D) we did not find any PIGA, PIGT or HLA mutation. Finally, myeloid gene mutations analysis revealed the presence of a subclonal ASXL1 mutation in 1/10 patients in the GPI(-) fraction. Of note, this patient (UPN 1) had older age and showed 12 different somatic PIGA hits. This finding is probably explicable with the scenario of PIGA as the initial ancestral event accompanied by secondary mutations previously shown by our group as occurring in 10% of PNH cases in the course of disease evolution.[7-8]
In summary, somatic HLA class I/II mutations can be found in patients with PNH. HLA mutations can occur in GPI(+) cells in subclonal fashion but also in GPI(-) cells. The latter clonal mosaicism indicates that various mechanisms of immune escape may play a role. Subclonal HLA mutations may impact the immune pressure on PNH clone dynamics, reflecting an alternative immune escape pathway in patients without PNH clone. (Fig.1E) In addition, detection of occasional "myeloid" hits suggests that various modes of PNH clone maintenance and expansion may be operative. We will present at ASH analysis of a full cohort of these patients including properly powered clinical correlations.
Maciejewski:Novartis, Roche: Consultancy, Honoraria; Alexion, BMS: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal